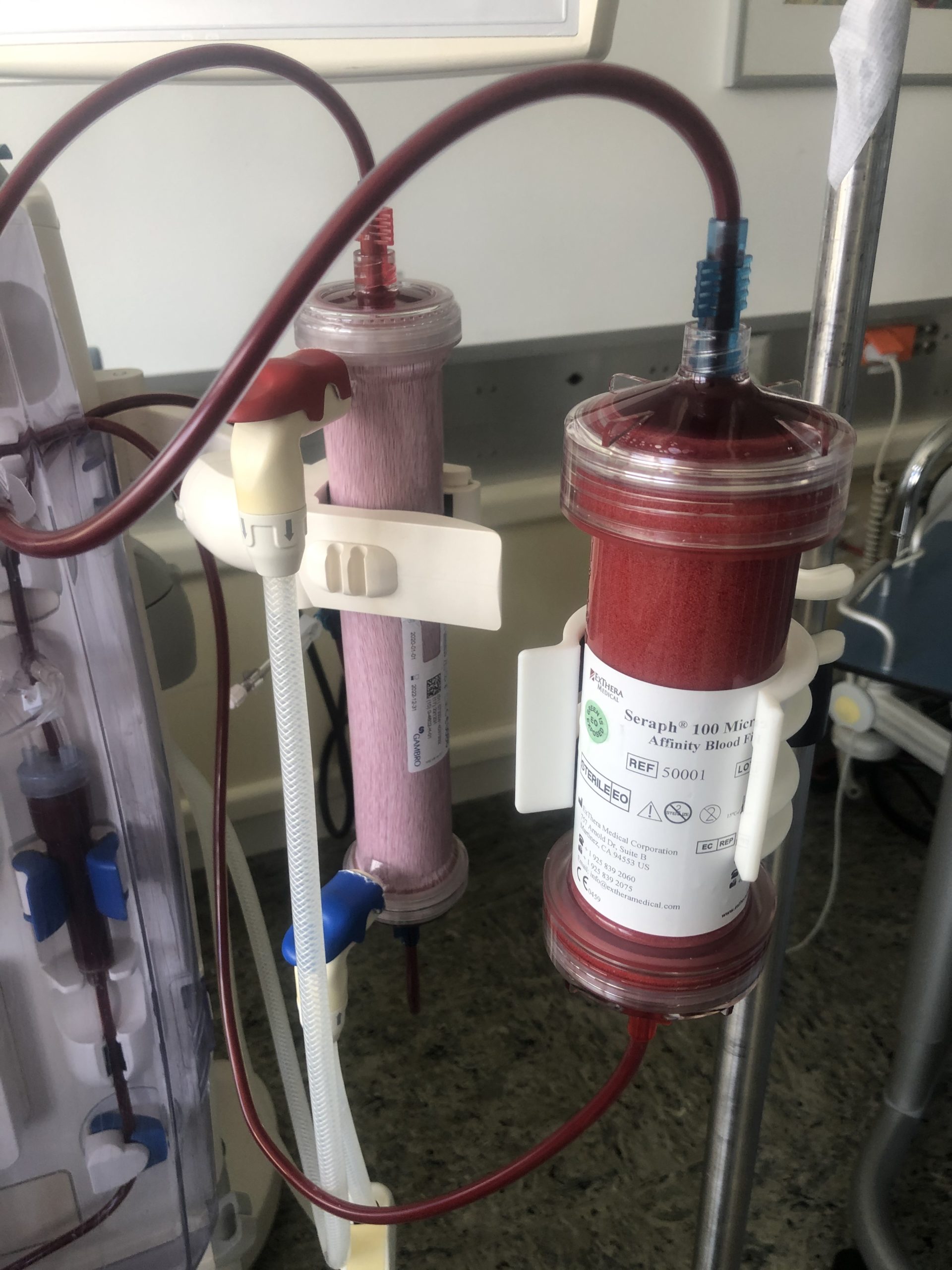
Seraph 100 Receives FDA Approval
The US Food and Drug Administration approved the Seraph 100 on the 17th April 2020 as a specific response to COVID-19. At this time there is no other blood purification therapy known to bind and remove blood-borne SARS-CoV-2 virus/RNA while also improving the vital signs of patients.
The Seraph100 has been used to treat patients in major European and US hospitals and has seen increasing repeat orders and new hospitals enrolling over recent weeks. Early results are very encouraging, with patients showing lower blood pathogen levels and improvements in mean arterial pressure and oxygen saturation.
ExThera Medical’s President and CEO Bob Ward commented: “We have confidence that Seraph 100 treatment alone or in combination with available anti-viral drugs and vaccines could help curb the current epidemic and save lives. We also have encouraging evidence, based on all patients treated to date, that Seraph improves lung function and because pneumonia or ‘ARDS’ kills people with COVID-19, we believe Seraph’s ability to reduce the virus in the bloodstream, combined with its ability to remove so-called sepsis mediators known to degrade capillary function in the lungs and other organs, may be a powerful combination when treating COVID-19 and other viral infections”.
Professor Jan T. Kielstein, Director of Nephrology, Rheumatology and Blood Purification at Academic Teaching Hospital Braunschweig, Germany is leading the deployment of the Seraph100 in Europe. He said: “We are pleased to see general improvement in the health of COVID-19 patients during and after treatment with Seraph 100. Treatment with this device has immediate and sustained effects on vital signs and laboratory parameters. Critically ill patients tolerate it very well in terms of cardiovascular stability. Aside from reducing pathogens in the blood, Seraph 100 has other features that that could benefit COVID-19 patients.”
The aim of the public private partnership between the Gorta Group and ExThera Medical is to ensure that world class medical devices are made accessible in a timely manner and at an affordable cost in lower and middle income countries.
CEO of the Gorta Group, Ray Jordan, said “This partnership will enable us to save lives in Africa by strengthening public health systems ability to respond to COVID-19. Regrettably over the years I have seen people in lower and middle-income countries die because of lack of access to essential health care and increasingly due to anti-microbial resistance. This partnership with ExThera Medical will change the way things are done. ExThera’s commitment to simultaneously deploy the Seraph 100 globally into both higher income country healthcare systems at market prices and humanitarian led programs at a significant discount should be a beacon for others to follow.”